By Dr. Susan E. Brown, PhD
Science has learned some amazing things about nutrients for bone health and osteoporosis in recent years. The outmoded thinking that says bone nutrition is all about calcium has been shown by research to be an oversimplification at best. Yes, calcium is critical for bone health — but our bones are so much more complex than we ever imagined!

Bones play a number of surprising roles in the body, from regulating blood calcium and blood sugar to storing needed minerals to supporting and protecting the body’s organs and tissues.
Getting the full complement of nutrients for bone health is essential for overall health. But it’s especially important for women in midlife to recognize the value of bone-building nutrients, because that’s when women start to lose bone mass. Perhaps you’ve been diagnosed with osteoporosis or told you have osteopenia and are wondering if there are nutrients for osteoporosis that can help you fend off a fracture. Or perhaps you’ve broken a bone and want to know what nutrients for bone healing can speed your recovery. We’ll look beyond the “usual suspects” and highlight nutrients that support bone health so that whatever you’re looking for — whether it’s nutrition for osteopenia or bone-healing nutrients for osteoporotic fractures — you’ll know what to look for and where to find it.
Calcium
Your body contains more calcium than any other mineral, making up about 2% of your total adult body weight. It’s stored in your bones and teeth, and the skeleton itself is a reserve of calcium. Calcium is vital to maintaining heart rhythm, muscle function, blood clotting and many enzymes, so it’s crucial that you have what you need — and if your body doesn’t get the calcium it needs from food or supplementation, it takes it from your skeleton, which weakens your bones.
Getting enough calcium isn’t as simple as adding more to your diet by eating cheese or yogurt.
Whether you can absorb the calcium in your diet is just as important, if not more important, than eating enough of it. And there are a lot of nutritional factors influencing absorption: for instance, calcium absorption is highly dependent on other nutrients for bone health such as Vitamin D, magnesium and zinc, among others. So having too little of such companion nutrients can affect how much calcium you absorb. As an example, a person with inadequate Vitamin D absorbs 65% less calcium than someone who has adequate Vitamin D (at least 32 ng/ml). Not to mention the fact that without nutrients for bone health like phosphorus, magnesium and Vitamin K, bones are not as strong and flexible.
Magnesium
Magnesium assures the strength and firmness of bones and makes teeth harder. It’s one of the key nutrients needed to ensure calcium is both optimally absorbed and best utilized by the body. It’s also necessary for converting Vitamin D into its active form. What’s more, magnesium is required for over 300 biochemical reactions in the body. It’s found in nuts, leafy greens, beans and, best of all, dark chocolate.
The majority of the body’s reserves (60%) of magnesium are held in the bone, and the bones act as a storage reservoir, transferring magnesium to the blood in times of need.
Vitamin D3
Ranking high on the list of nutrients for bone health, Vitamin D — and specifically the hormone our body produces from Vitamin D — is the most important regulator of calcium absorption. People with low Vitamin D absorb 65% less calcium than those with adequate levels of this vitamin. Vitamin D is one of the few nutrients we make ourselves, simply by exposing skin to sunlight for 10 to 30 minutes a day. It’s an easy vitamin to get if you live in a sunny location — not so easy if you live in a northern state where it’s wintry half the year, which is why people who are housebound or who live in colder climates are encouraged to take supplements. Osteoporotic fractures are much more common in folks with low levels of Vitamin D, though fracture incidence can be dramatically reduced with Vitamin D supplementation.
Low levels of Vitamin D are also linked to the development of numerous diseases, including:
- Cancer
- Heart disease
- Diabetes
- Hypertension
- Depression
- Muscle weakness
- Dementia
- Autoimmune disease (such as MS)
Vitamin K
Emerging research is showing Vitamin K2 as MK-7 (menaquinone-7) to be one of the key nutrients for bone health, specifically for building bone strength and helping to prevent osteoporosis, protecting the heart and even reducing mortality. For example, K2 is involved in preventing fractures in postmenopausal women with osteoporosis. An analysis of the results of 19 different studies focusing on postmenopausal women with osteoporosis showed that Vitamin K2 plays a role in improvement of the vertebral bone mineral density and the prevention of fractures.
Vitamin K2 as MK-7 has also been shown to improve cardiovascular health in healthy postmenopausal women. Finally, in one recent study, participants who increased their Vitamin K1 and K2 dietary intakes over the nearly five-year follow-up period had a 43% and 45% reduced risk of overall mortality respectively compared to those whose intakes were unchanged or reduced. Those with increased Vitamin K2 intakes during follow-up had a 59% lower risk of death from cancer. Vitamin K is most often associated with leafy greens (kale is particularly high in this vitamin) but it’s also found in hard cheeses like manchego or Romano.
Zinc
Zinc helps produce the matrix of collagen protein threads upon which the all-important bone-forming calcium–phosphorus compound is deposited. It’s also necessary for the production of enzymes that degrade and recycle worn-out bits of bone protein. Zinc deficiency is problematic because it prevents full absorption of calcium. Low levels have been closely linked with the development of osteoporosis. Beans, nuts, shellfish and whole grains are good sources of zinc.
Manganese
Manganese is often overlooked among important nutrients for bone health — there’s not even an RDA for the trace element! However, research shows clearly that manganese is a co-factor in the formation of bone cartilage and bone collagen, as well as in bone mineralization.
Manganese deficiency can have serious consequences — it appears to increase bone breakdown while decreasing new bone mineralization — that can lead to osteoporosis. For example, in one study, blood levels of manganese in severely osteoporotic women were found to be just 1/4 those of non-osteoporotic women their same age — and it was the only significantly different variable out of 25 studied. Manganese is found in a lot of different foods — beans, nuts, rice, leafy vegetables, even coffee and tea.
Boron
The benefits of boron for bone health have only recently been discovered. Your body requires boron for proper metabolism and utilization of various bone-building factors, including calcium, magnesium, Vitamin D, estrogen and perhaps testosterone. Overall studies show boron has a mineral-conserving and estrogen-enhancing effect, especially among women with low magnesium intake. Boron is generally found in non-citrus fruits, nuts, grains and leafy greens.
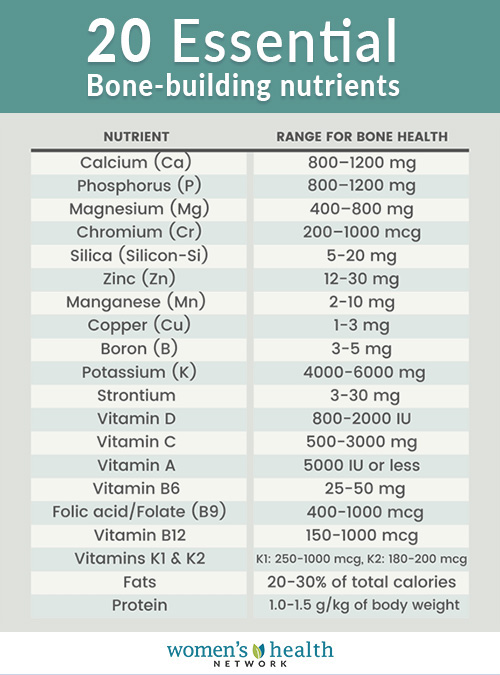
Chromium
As well as keeping insulin activity in the body efficient, chromium may protect bone by promoting the production of collagen by osteoblasts (our bone-building cells) and by moderating bone breakdown (resorption). Chromium may also improve bone health by raising blood levels of the hormone DHEA, which is thought to be important to the preservation of bone density among postmenopausal women. Grapes and grape juice, apples, beef, poultry and broccoli are all good sources of chromium.
Silica
High amounts of silica are found in the strongest body tissues, such as the arteries, tendons, ligaments, connective tissue, collagen, skin, nails, hair and teeth. In terms of its ranking among nutrients for bone health, it was found that bone collagen increases with silica supplementation and that the mineral is involved in strengthening the connective tissue matrix by cross-linking collagen strands. Furthermore, dietary silicon increases the rate of mineralization and high concentrations of silica are found in areas of active bone mineralization. Silica also combines with calcium in bone-building cells. Green beans, bananas, leafy greens and brown rice are all rich in silica.
Copper
Copper is a trace mineral essential to bone health maintenance. Although its role is not fully understood, a copper-containing enzyme called lysyl oxidase is known to help form collagen for bone and connective tissue and contributes to the strength of bone collagen fibrils. Also, copper is an important element of an antioxidant called superoxide dismutase that helps limit bone resorption — perhaps one reason why low levels of copper are associated with the development of osteoporosis. Copper is not common in a lot of foods, but leafy greens, dark chocolate and seafood are good sources.
Potassium
Potassium, along with sodium, helps to maintain critical fluid balance in the body. Potassium also helps bone in the form of alkalizing compounds that neutralize bone-depleting acids — which prevents too much calcium from being excreted in the urine. The prevention of excess calcium loss in the urine can be done through dietary potassium (think bananas, melons, citrus fruits, spinach, broccoli and cucumbers) or supplemental potassium, such as potassium bicarbonate and potassium citrate.
Vitamin C
Vitamin C is an antioxidant that protects bone cells from oxidative damage. Most people know that it’s present in citrus fruits, but you can also get it from tomatoes and tomato juice, peppers, strawberries, brussels sprouts and potatoes. It is essential for bone health in multiple ways:
- Vitamin C helps form collagen. Bone mineral is laid over a protein matrix, collagen, which is the connective tissue of cartilage and bone. It makes up 30% of our bones.
- Vitamin C may stimulate bone-building cells, enhance calcium absorption and promote Vitamin D’s effect on bone metabolism.
- Vitamin C is responsible for the synthesis and optimal function of adrenal steroid hormones, which are vital in bone health. These hormones are especially important during perimenopause and menopause.
- Vitamin C is the most abundant water-soluble antioxidant in the body.
- Vitamin C is a potent anti-inflammatory that decreases bone-damaging immune system pro-inflammatory compounds.
- Vitamin C chaperones toxins stored in bone out of the body.

Vitamin A
Vitamin A plays a significant role in developing osteoblasts, the bone-building cells that lay down new bone. It’s available through a wide range of dietary sources, both animal and vegetable. Vitamin A deficiency inhibits calcium absorption and metabolism, increasing the risk of poor bone growth. In general, low Vitamin A levels are associated with osteoporosis and increased fracture risk. We recommend the retinoid forms of Vitamin A.
Vitamin B6
Vitamin B6 plays an important yet indirect role in bone metabolism in the following ways:
- Vitamin B6 is needed for hydrochloric acid (HCI) production by the stomach which is necessary for calcium absorption.
- Adrenal functioning needs Vitamin B6. Several hormones are then released by the adrenal glands, some of which aid in maintaining proper mineral balance.
- Vitamin B6 is a cofactor in the enzymatic cross-linking of collagen strands. This means that it increases the strength of connective tissue.
- Vitamin B6 plays a role in the breakdown of homocysteine, a metabolite of an amino acid that interferes with collagen cross-linking and can cause defective bone matrix and osteoporosis. It also contributes to the development of heart disease. Vitamin B6 and folic acid work together to prevent a build-up of homocysteine. Fish, chicken, beans, bananas and oats are all good sources of Vitamin B6.
Folic acid/folate (Vitamin B9)
Folate is necessary for the proper processing of homocysteine. Its role is specifically in the detoxification of the metabolite. Vitamin B9’s role in homocysteine regulation is essential for osteoporosis and atherosclerosis prevention. The best sources of folate are leafy green vegetables, broccoli and beans.
Vitamin B12
Another B vitamin that plays a role in the detoxification of homocysteine is Vitamin B12, which is essential for red blood cell production. Osteoblasts need B12 to function. This vitamin is often found in fish or meat products, which makes it problematic for vegans and vegetarians unless they supplement — and because low B12 levels can cause pernicious anemia (low red blood cell count), it’s generally recommended that people who don’t eat meat take supplements.
Research has found that overall deficiency in B12 is linked with osteoporosis.
Fats
Although we Americans are consuming too much fat in our diets, there are certain fats that are important for our health called essential fatty acids. In fact, our bodies require these fats. Essential fatty acids are not produced by the body and are only obtained through diet or supplementation. They are important for nerve functioning, hormone production, maintenance and function of the brain, and everyday energy production. Essential fatty acids also play a role in bone health. These fats are needed for calcium metabolism and are essential components of all membranes, such as cartilage and bone. EFAs increase calcium absorption from the intestines, regulate and reduce calcium excretion in the urine, and increase calcium deposition in bone. Omega-3 specifically serves as a potent anti-inflammatory to protect bones from cytokine damage.
Protein
Protein promotes intestinal absorption of calcium and serves as a major building block for bone. Protein even makes up about one-third to one-half of our bone. Additionally, it has been found that protein malnutrition weakens bone. The most obvious sources of protein are meat, eggs and dairy, but whole grains, nuts and beans offer vegetable proteins as well.
Bottom line on nutrients for bone health and osteoporosis
Ideally, we would get the optimal supply of supplemental nutrients from the food we eat. But for most of us, that’s just not possible — even when we’re doing our very best to eat well. Plus, most people don’t realize how critical specific nutrients are for the absorption and utilization of other nutrients. Without this synergistic effect, your body can’t absorb certain nutrients and won’t get the benefits.
To get the right amounts of the top nutrients for bone health every day, you may want to use a nutritional supplement in addition to enjoying a healthy diet. When choosing your supplements, go with a medical-grade multivitamin/mineral formulated specifically for bone health, and make sure it contains balanced, therapeutic levels of these nutrients in their most bioavailable forms.
Our exclusive Better Bones Builder is formulated with ideal amounts of Vitamin D, calcium and magnesium and all other essential bone-building vitamins and minerals at therapeutic levels, along with nutrients to optimize absorption and bone-building support.
References
US DHHS. 2004. Bone health and osteoporosis: A report of the Surgeon General. Chapter 1: A public health approach to promote bone health. URL: https://www.surgeongeneral.gov/library/bone-health/chapter_1.html (accessed 05.28.2008).
Wright, J., et al. 2003. Dietary intakes of ten key nutrients for public health, United States: 1999–2000. Adv. Data, (334), 1–4. URL (PDF): https://www.cdc.gov/nchs/data/ad/ad334.pdf (accessed 05.28.2008).
Morgan, K., et al. 1985. Magnesium and calcium dietary intakes of the US population. J. Am. Coll. Nutr., 4 (2), 195–206. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/4019942 (accessed 05.28.2008).
Brown, J. 2005. Nutrition Now. Belmont, CA: Wadsworth Publishing.
Pennington, J., et al. 1986. Mineral content of foods and total diets: The Selected Minerals in Foods Survey, 1982 to 1984. J. Am. Diet. Assoc., 86 (7), 876–891. URL: https://www.ncbi.nlm.nih.gov/pubmed/3722652 (accessed 05.28.2008).
Morgan, K., et al. 1985. Magnesium and calcium dietary intakes of the US population. J. Am. Coll. Nutr., 4 (2), 195–206. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/4019942 (accessed 05.28.2008).
Lakshmanan, F., et al. 1984. Magnesium intakes, balances, and blood levels of adults consuming self-selected diets. Am. J. Clin. Nutr., 40 (6 Suppl.), 1380–1389. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/6507359 (accessed 05.28.2008).
Moshfegh, A., et al. 2005. What we eat in America, NHANES 2001–2002: Usual nutrient intakes from food compared to Dietary Reference Intakes. USDA, Agricultural Research Service. URL: https://www.ars.usda.gov/SP2UserFiles/Place/12355000/pdf/usualintaketables2001-02.pdf (accessed 06.17.2008).
Kumpulainen, J. 1992. Chromium content of foods and diets. Biol. Trace Elem. Res., 32 (1–3), 9–18. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/1375091 (accessed 10.19.2009).
Jugdaohsingh, R., et al. 2002. Dietary silicon and absorption. Am. J. Clin. Nutr., 75 (5), 887–893. URL: https://www.ajcn.org/cgi/content/full/75/5/887 (accessed 05.28.2008).
Freeland–Graves, J., et al. 1988. Metabolic balance of manganese in young men consuming diets containing five levels of dietary manganese. J. Nutr., 118 (6), 764–773. URL: https://jn.nutrition.org/cgi/reprint/118/6/764 (accessed 05.28.2008).
Klevay, L. 1979. Evidence of dietary copper and zinc deficiencies. JAMA, 241, 1917–1918. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/430773 (accessed 01.28.2010).
Nielsen, F., et al. 1987. Effect of dietary boron on mineral, estrogen, and testosterone metabolism in postmenopausal women. FASEB J., 1 (5), 394–397. URL: https://www.fasebj.org/cgi/reprint/1/5/394 (accessed 05.13.2008).
Hajjar, et al. 2001. Impact of diet on blood pressure and age-related changes in blood pressure in the US population: Analysis of HNANES III. Arch. Intern. Med., 161 (4), 589–593. URL: https://archinte.ama-assn.org/cgi/content/full/161/4/589 (accessed 05.28.2008).
Holick, M. 2007. Vitamin D deficiency. New Eng. J. Med., 357 (3), 266–281. URL: https://content.nejm.org/cgi/content/full/357/3/266 (accessed 05.28.2008).
Kimlin, M., et al. 2007. Location and vitamin D synthesis: Is the hypothesis validated by geophysical data? J. Photochem. Photobiol., 86 (3), 234–249. URL: https://www.ncbi.nlm.nih.gov/pubmed/17142054 (accessed 05.20.2008).
PDRHealth. [No date listed]. Vitamin C | Herbal remedies, supplements | PDRHealth. URL: https://www.pdrhealth.com/drugs/altmed/altmed-mono/?contentFileName=ame0173.xml&contentName=Vitamin+C&contentId=336 (accessed 05.13.2008).
Serfontein, W., et al. 1984. Vitamin B6 revisited. Evidence of subclinical deficiencies in various segments of the population and possible consequences thereof. S. Afr. Med. J., 66 (12), 437–440. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/6385307 (accessed 05.13.2008).
Song, W., et al. 2005. Serum homocysteine concentration of US adults associated with fortified cereal consumption. J. Am. Coll. Nutr., 24 (6), 503–509. URL: https://www.jacn.org/cgi/content/full/24/6/503 (accessed 06.17.2008).
Botto, L., & Yang, Q. 2000. 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: A HuGE review. Am. J. Epidem., 151 (6), 862. URL: https://www.ajcn.org/cgi/reprint/87/3/734 (accessed 07.21.2008).
McBride, J. 2000. B12 Deficiency may be more widespread than thought — August 1, 2000 — News from the USDA Agricultural Research Service. URL: https://www.ars.usda.gov/IS/pr/2000/000802.htm (accessed 06.17.2008).
Booth, S., & Suttie, J. 1998. Dietary intake and adequacy of vitamin K. J. Nutr., 128 (5), 785–788. Review. URL: https://jn.nutrition.org/cgi/content/full/128/5/785 (accessed 05.28.2008).
National Academy of Sciences. Institute of Medicine. Food and Nutrition Board. 2002. Through the United States Department of Agriculture Food and Nutrition Information Center website. Dietary Reference Intakes for individuals. PDF: https://iom.edu/en/Global/News%20Announcements/~/media/Files/Activity%20Files/Nutrition/DRIs/DRISummaryListing2.ashx (accessed 02.02.2010).
Larsen, T., et al. 2000. Whole small fish as a rich calcium source. Br. J. Nutr., 83 (2), 191–196. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/10743499 (accessed 05.06.2008).
Hansen, M., et al. 1998. Calcium absorption from small, soft-boned fish. J. Trace Elem. Med. Biol., 12 (3), 148–154. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/9857327 (accessed 05.06.2008).
Heaney, R., & Weaver, C. 1990. Calcium absorption from kale. Am. J. Clin. Nutr., 51, 656–657. URL: https://www.ajcn.org/cgi/reprint/51/4/656 (accessed 05.06.2008).
Weaver, C., et al. 1999. Choices for achieving adequate dietary calcium with a vegetarian diet. Am. J. Clin. Nutr., 70 (Suppl.), 543S–548S. URL: https://www.ajcn.org/cgi/content/full/70/3/543S (accessed 01.28.2010).
Heaney, R., & Weaver, C. 2003. Calcium and vitamin D. Endocrinol. Metab. Clin. N. Am., 32 (1), 181–194, vii–viii. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/12699298 (accessed 05.20.2008).
Murray, M., & Pizzorno, J. 1998. Encyclopedia of Natural Medicine, 459. Roseville, CA: Prima Publishing.
Brown, S. 2008. Vitamin D and fracture reduction: An evaluation of the existing research. Alt. Med. Rev., 13 (1), 21–33. URL (PDF): https://www.thorne.com/altmedrev/.fulltext/13/1/21.pdf (accessed 05.22.2008).
Heaney, R., & Weaver, C. 2003. Calcium and vitamin D. Endocrinol. Metab. Clin. N. Am., 32 (1), 181–194, vii–viii. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/12699298 (accessed 05.20.2008).
Heaney, R., et al. 2003. Calcium absorption varies within the reference range for serum 25–hydroxyvitamin D. J. Am. Coll. Nutr., 22 (2) 142–146. URL: https://www.jacn.org/cgi/content/full/22/2/142 (accessed 05.22.2008).
Randall, T. 1992. Longitudinal study pursues questions of calcium, hormones, and metabolism in life of skeleton. JAMA, 268 (17), 2357–2358.
Sakhaee, K., et al. 2004. Stone forming risk of calcium citrate supplementation in healthy postmenopausal women. J. Urol., 172 (3), 958–961. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/15311008 (accessed 05.06.2008).
Wabner, C., & Pak, C. 1992. Modification by food of the calcium absorbability and physiochemical effects of calcium citrate. J. Am. Coll. Nutr., 11, 548–552. URL: (abstract): https://www.ncbi.nlm.nih.gov/pubmed/1452953 (accessed 05.06.2008).
Heller, H., et al. 2000. Pharmacokinetic and pharmacodynamics comparison of two calcium supplements in postmenopausal women. J. Clin. Pharmacol., 40 (11), 1237–1244. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/11075309 (accessed 05.28.2008).
Peck W., et al. 1991. Physician’s Resource Manual on Osteoporosis. Washington, DC: National Osteoporosis Foundation.
Food and Nutrition Board, Institute of Medicine. 1997. Phosphorus. In Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride, 146–189. Washington, DC: National Academy Press. URL: https://www.nap.edu/books/0309063507/html/index.html (accessed 05.20.2008).
Worthington–Roberts, B. 1981. Contemporary Developments in Nutrition, 240–253. St. Louis, MO: Mosby Co.
Linkswiler, H., et al. 1981. Protein-induced hypercalciuria. Fed. Proc., 40 (9), 2429–2433. URL: https://www.ncbi.nlm.nih.gov/pubmed/7250387 (accessed 05.06.2008).
Medalle, R., et al. 1976. Vitamin D resistance in magnesium deficiency. Am. J. Clin. Nutr., 29, 854–858. URL: https://www.ajcn.org/cgi/reprint/29/8/854 (accessed 05.12.2008).
Rude, R., et al. 2006. Reduction of dietary magnesium by only 50% in the rat disrupts bone and mineral metabolism. Osteoporos. Int., 17 (7), 1022–1032. URL (abstract) https://www.ncbi.nlm.nih.gov/pubmed/16601920 (accessed 05.12.2008).
Rude, R., et al. 2005. Dietary magnesium reduction to 25% of nutrient requirement disrupts bone and mineral metabolism in the rat. Bone, 37 (2), 211–219. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/15923157 (accessed 05.12.2008).
Rude, R., et al. 1999. Magnesium deficiency-induced osteoporosis in the rat: Uncoupling of bone formation and bone resorption. Magnes. Res., 14 (4), 257–267. URL: (abstract): https://www.ncbi.nlm.nih.gov/pubmed/10612083 (accessed 05.12.2008).
Iseri, L., & French, J. 1984. Magnesium: Nature’s physiologic calcium blocker. Am. Heart J., 108, 188–193.
Cohen, L., & Kitzes, R. 1981. Infrared spectroscopy and magnesium content of bone mineral in osteoporotic women. Israel J. Med. Sci., 17, 1123–1125. URL:
Seelig, M. 1980. Magnesium Deficiency in the Pathogenesis of Disease. New York: Plenum Press. URL: https://www.mgwater.com/Seelig/Magnesium-Deficiency-in-the-Pathogenesis-of-Disease/preface.shtml (accessed 05.12.2008).
Gaby, A., & Wright, J. 1988. Nutrients and bone health. Health World, 29–31.
Hegsted, D. 1967. Mineral intake and bone loss. Fed. Proceedings, 26 (6), 1747–1763.
Shils, M. 1973. “Magnesium.” In Modern Nutrition in Health and Disease, ed. R. Goodhart & M. Shils. Philadelphia: Lea & Febiger.
Pennington, J. 1996. Intakes of minerals from diets and foods: Is there a need for concern? J. Nutr., 126 (9 Suppl.), 2304S–2308S. URL: https://jn.nutrition.org/cgi/reprint/126/9_Suppl/2304S (accessed 05.13.2008).
Hunt, C., & Johnson, L. 2006. Magnesium requirements: New estimations for men and women by cross-sectional statistical analyses of metabolic magnesium balance data. Am. J. Clin. Nutr., 84 (4), 843–852. URL: https://www.ajcn.org/cgi/content/full/84/4/843 (accessed 05.13.2008).
Food & Nutrition Board, Institute of Medicine. 1997. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press.
Martin, J., et al. 2006. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. Diabetes Care, 29 (8), 1826–1832. URL: https://care.diabetesjournals.org/content/29/8/1826.long (accessed 10.20.2009).
Frauchiger, M., et al. 2004. Effects of acute chromium supplementation on postprandial metabolism in healthy young men. J. Am. Coll. Nutr., 23 (4), 351–357. URL: https://www.jacn.org/cgi/content/full/23/4/351 (accessed 10.20.2009).
McCarty, M. 1995. Anabolic effects of insulin on bone suggest a role for chromium picolinate in preservation of bone density. Med. Hypotheses, 45 (3), 241–246. URL: https://www.ncbi.nlm.nih.gov/pubmed/8569546 (accessed 10.20.2009).
Evans, G., et al. 1995. Chromium picolinate decreases calcium excretion and increases dehydroepiandrosterone (DHEA) in postmenopausal women. FASEB J., 9, A449. [As quoted in Lamson, D., & Plaza, S. 2002. The safety and efficacy of high-dose chromium. Altern. Med. Rev., 7 (3), 218–235. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/12126463 (accessed 10.20.2009).]
Anderson, R. “Chromium in Health and Disease.” Council for the Advancement of Diabetes Research and Education (CADRE) Chromium Summit. April 2003. Boston, Massachusetts.
Bae, Y., et al. 2008. Short-term administration of water-soluble silicon improves mineral density of the femur and tibia in ovariectomized rats. Biol. Trace Elem. Res.,124 (2), 157–163. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/18438624 (accessed 05.13.2008).
Davies, S. & Stewart, A. 1987. Nutritional Medicine: The Drug-free Guide to Better Family Health. London/Sydney: Pan Books.
Carlisle, E. 1975. Silicon with the osteoblast, the bond-forming cell. Fed. Proc., 34, 927.
Carlisle, E. 1970. A relationship between silicon and calcium in bone formation. Fed. Proc., 29, 265.
Anderson, J. 1999. Plant-based diets and bone health: Nutritional implications. Am. J. Clin. Nutr., 70 (3) (Suppl,), 539S–542S. URL: https://www.ajcn.org/cgi/content/full/70/3/539S (accessed 05.28.2008).
Chen, F., et al. 1994. Estimates of trace element intakes in Chinese farmers. J. Nutr., 124, 196–201.
Anasuya, A., et al. 1996. Fluoride and silicon intake in normal and endemic fluorotic areas. J. Trace Elem. Med. Biol., 10 (3), 149–155. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/8905558 (accessed 05.28.2008).
Gullberg, B., et al. 1997. World-wide projections for hip fracture. Osteoporos. Int., 7, 407–413. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/9425497 (accessed 05.28.2008).
Kamen, B., et al. 1984. Osteoporosis: What It Is, How to Prevent It, How to Stop It, 222. NY: Pinnacle Books.
Jugdaohsingh, R., et al. 2002. Dietary silicon and absorption. Am. J. Clin. Nutr., 75 (5), 887–893. URL: https://www.ajcn.org/cgi/content/full/75/5/887 (accessed 05.28.2008).
Kimmel, P., et al. 1992. Zinc nutritional status modulates the response of 1,25-dihydroxycholecalciferol to calcium depletion in rats. J. Nutr., 122 (7), 1576–1581. URL: https://jn.nutrition.org/cgi/reprint/122/7/1576 (accessed 05.13.2008).
Teller, E., et al. 1987. Zinc (Z) nutritional status modulates the 1,25(OH)2D(125) response to low calcium (LC) diet (D). Kidney Int., 31, 358.
Hambidge, M. 2000. Human zinc deficiency. J. Nutr., 130 (5), 1344S–1349S. URL: https://jn.nutrition.org/cgi/content/full/130/5/1344S (accessed 07.21.2008).
Johtatsu, T., et al. 2007. Serum concentrations of trace elements in patients with Crohn’s disease receiving enteral nutrition. J. Clin. Biochem. Nutr., 41 (3), 197–201. URL: https://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=18299716 (accessed 05.13.2008).
Hendricks, K. 1990. Zinc and inflammatory bowel disease. Nutr. Report, 66.
Atik, S. 1983. Zinc and senile osteoporosis. J. Am. Ger. Soc., 31 (12), 790–791. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/6655182 (accessed 05.13.2008).
Greger, J. 1998. Dietary standards for manganese: Overlap between nutritional and toxicological studies. J. Nutr., 128 (2), 368S–371S. URL: https://jn.nutrition.org/cgi/content/full/128/2/368S (accessed 05.13.2008).
Raloff, J. 1986. Reasons for boning up on manganese. [Review.] Science News, 130, 199.
Schwartz, R., et al. 1986. Apparent absorption and retention of Ca, Cu, Mg, Mn, Zn from a diet containing bran. Am. J. Clin. Nutr., 43 (3), 444–445. URL: https://www.ajcn.org/cgi/reprint/43/3/444 (accessed 05.13.2008).
Pennington, J., & Young, B. 1991. Total Diet Study nutritional elements 1982–1989. J. Am. Diet. Assoc., 91 (2), 179–183. URL (abstract): https://www.ncbi.nlm.nih.gov/pubmed/1991931 (accessed 05.13.2008).












