$29.95
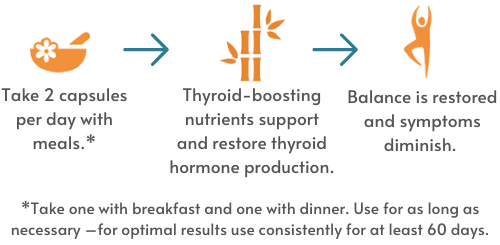
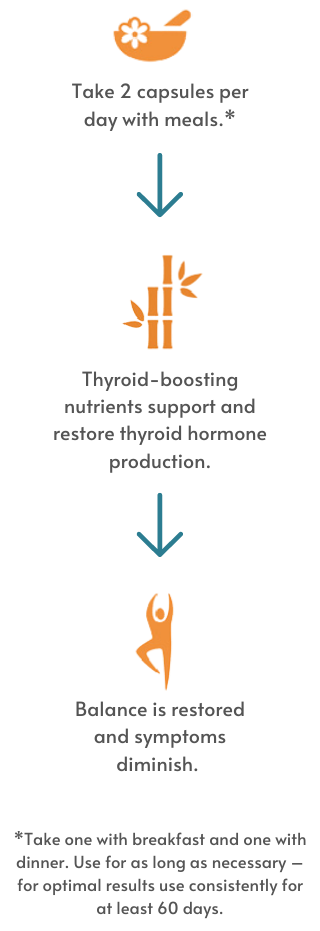
This completely natural supplement helps relieve symptoms of subclinical hypothyroidism, or underactive thyroid including loss of energy, hair loss, weight gain, fuzzy thinking, skin changes, and sensitivity to cold temperatures. Your thyroid hormones (T4 and T3) affect how other hormones in your body behave. The thyroid itself is deeply involved in metabolism and how your body uses energy. T-Balance Plus is formulated to boost energy and support healthy cell metabolism in the thyroid gland and in other areas where thyroid hormone receptors and thyroid hormone conversion activity also occur.
T-Balance Plus contains:
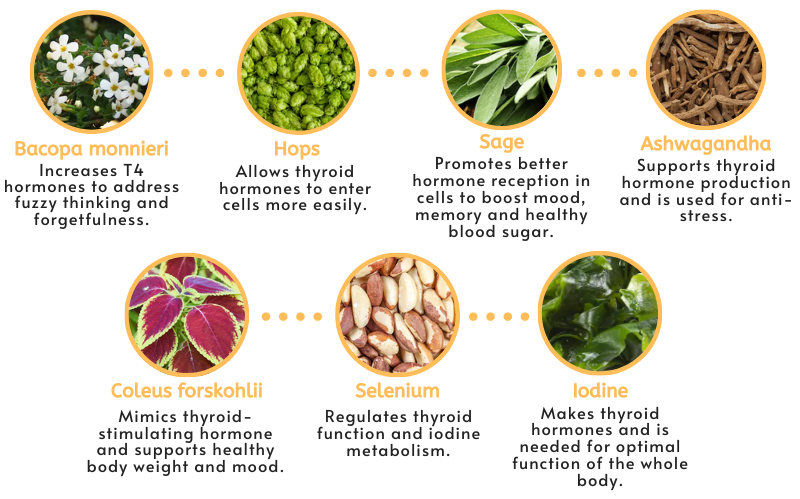
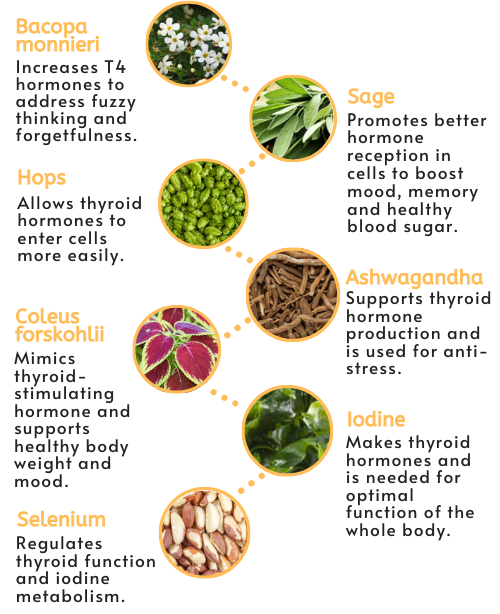
- Bacopa monnieri — used for thousands of years in ayurvedic medicine, this aquatic plant increases T4 hormone concentrations and may help address fuzzy thinking and forgetfulness.
- Hops — extracts have actions that allow thyroid hormones to enter cells more easily.
- Sage — this herb has anti-inflammatory and antioxidant effects, with phytochemicals that promote better hormone receptor function and support improved mood, memory, and healthy blood sugar balance.
- Ashwagandha — an ayurvedic herb widely used for its anti-stress and calming properties, it supports production of thyroid hormones and helps correct imbalances in the nervous, endocrine and immune systems.
- Coleus forskohlii — a phytochemical in this extract mimics the effect of thyroid-stimulating hormone to enhance iodine uptake and thyroid hormone production. Studies show it has supportive effects on the immune system, body weight, and for depression.
- Iodine — this potent antioxidant synthesizes thyroid hormones and is needed for optimal function of every organ and cell in the body.
- Selenium — proper levels of this micronutrient are necessary to regulate thyroid function and iodine metabolism.
How will T-Balance Plus help you?
- Provides a boost in energy.
- Balances the thyroid gland and supports normal thyroid function.
- Helps maintain thyroid hormone production.
- Helps support healthy thyroid cell metabolism.
Our T-Balance Plus is:
- A precisely proportioned blend of trace elements and traditional and cutting-edge herbs.
- Made without preservatives, sugar, artificial flavoring, dyes, or coloring of any kind.
- Laboratory-assayed to ensure quality — the same rigorous standard used for pharmaceutical drugs.
|
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
|
Reviews for T-Balance™ Plus
Loading product reviews

Ingredients for T-Balance™ Plus